Patients who had IVC (inferior vena cava) filters implanted in their bodies are now filing legal claims after their filters fractured, causing them severe harm. IVC filters are meant to minimize the risk of blood clots for those who can’t take blood thinners. However, Cook IVC filter lawsuits claim these filters caused severe health complications in some patients.
Claimants allege their IVC filters fractured and punctured tissues and veins after they moved to other body parts. These devices damaged the heart, lungs, and other body parts. IVC filter lawsuits also allege that some filters, after migrating, were difficult to remove and caused organ damage and organ perforation.
What Are Cook IVC Filters and Why Were They Used?
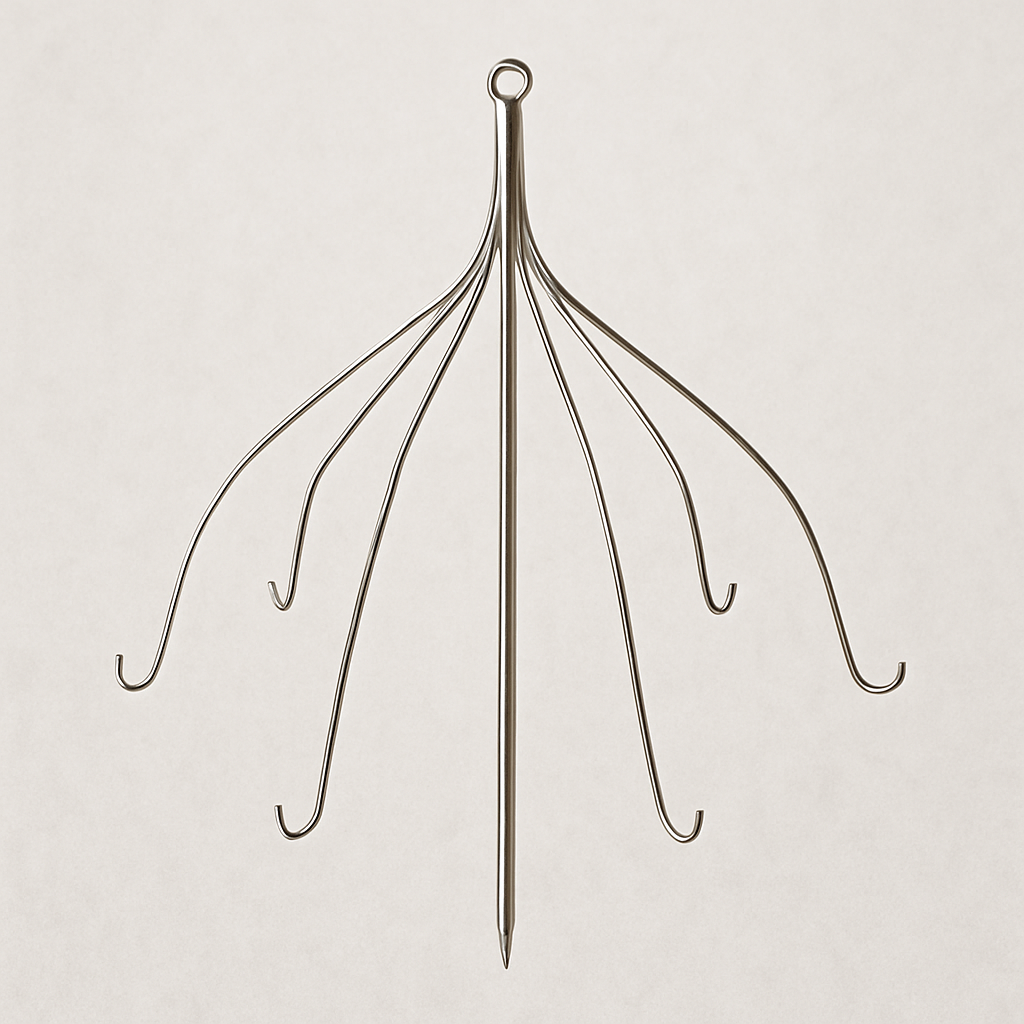
Inferior vena cava filters are small, cage-like medical devices that are implanted in the inferior vena cava to prevent blood clots from entering the lungs.
The inferior vena cava is the primary blood vessel transporting blood from the lower part of the body to the heart. Inferior vena cava filters are periodically placed in people at risk of developing pulmonary embolism (blood clots in the lungs) when doctors feel that anticoagulant therapy is ineffective or can’t be used.
These medical devices are designed to be retrievable, which means doctors should be able to remove them once the risk of developing pulmonary embolism subsides.
Although these devices are life-saving, they come with some potential health risks. Some potential IVC filter complications include filter migration, fracture, perforation of the vena cava, and even difficulties with removal.
Understanding the Allegations Against Cook Medical
Cook Medical and C.R. Bard are named as defendants in the largest portion of IVC filter injury claims related to defective IVC filters. Both corporations settled thousands of defective Cook IVC filter claims, but more claims are still ongoing following years of legal back-and-forth.
Additionally, plaintiffs have filed other IVC filter injury claims against other manufacturers, including Argon, ALN, CORDIS, and Boston Scientific. As of July 2025, none of these legal actions are part of any MDL (multidistrict litigation).
IVC filter lawsuits argue that the defendants didn’t adequately warn the public about the potential health complications attributed to their IVC filters, and that the defendants were irresponsible in the labelling, licensing, marketing, warning, and sale of IVC filters.
The lawsuits also claim that IVC filters can cause severe harm, complications, and conditions, including:
- Potential IVC migration
- Potential IVC perforation
- Potential inability to retrieve or remove the medical device
- Potential IVC embolization
Many claimants also allege emotional distress after IVC filters remain inside their bodies.
Common Injuries Linked to Defective IVC Filters
Several signs indicate a problem with your IVC blood clot filter. Contact your doctor immediately if you suspect something is wrong with your filter.
Some of the warning symptoms that there’s a problem with a Cook IVC blood clot filter include:
- Shortness of breath
- Nausea
- Confusion
- Lightheadedness
- Swollen legs
- Chest and neck pain
- Rapid heartbeat
- Upper body pain
Reported IVC blood clot filter complications include:
- Filter migration or fracture: IVC filters may break apart or move to your lungs or heart, causing severe embolism.
- Filter-related thrombosis: Blood clots may become trapped in the filter, leading to severe edema, pain, and even ulcers.
- Vein perforation: The IVC filter’s legs may penetrate the inferior vena cava walls, causing devastating damage.
- Elevated DVT (deep vein thrombosis) risks: Deep vein thrombosis occurs when blood clots form in deep veins, especially in the pelvis or legs. That may cause chronic pain and swelling in the legs.
FDA Warnings and Safety Concerns
The FDA issued a formal warning to C.R. Bard in July 2015 regarding violations in two Bard facilities. The violations included infractions of quality system regulations and misbranding. Also, the FDA accused the company of producing, distributing, and selling unapproved medical devices.
Furthermore, in 2010, the FDA issued a safety communication instructing the retrieval of IVC blood filters when they are no longer required, preferably within 29 to 54 days after the original implantation. The FDA updated its communication in 2014, emphasizing the importance of retrieving IVC blood clot filters within the specified timeframe.
Additionally, the FDA has issued several recalls of IVC devices for various reasons.
Recalled IVC products include:
- Greenfield: In 2005, the FDA recalled 33,000 units of Boston Scientific’s Greenfield IVC filters due to risks of components detaching and possibly causing blood clots.
- Cordis Optease: In 2013, the FDA recalled 33,000 units of Cordis Optease IVC filters due to a printing error. The error caused an upside-down installation of the filters, necessitating a second surgery to correct the issue.
- Bard Denali: In 2025, the FDA recalled 1,183 units of C.R. Bard’s Denali IVC filters because of mislabelling.
- Cook Celect Vena Cava Filter: The 2019 Cook Medical filter recall affected Cook Celect Vena Cava filters. The company stated they needed to update the instructions for use on the label of the filters. This recall followed a 2014 warning letter from the FDA, which asserted that Cook’s packing, manufacturing, installation, and storage procedures didn’t meet federal standards. In 2019, 91,721 units of Cook Medical’s Gunther Tulip filters were also recalled to update usage instructions.
It’s imperative to note that a recall isn’t necessary to file a blood clot filter lawsuit for injury or damage. Many of the IVC filter lawsuits were filed because the plaintiffs were patients whose IVC blood clot filters remained in their bodies past the recommended 29- to 54-day timeframe. These claimants didn’t know that doctors should have retrieved their filters.
Lawsuit Status: Class Actions, Settlements, and Verdicts
Thousands of plaintiffs have filed claims against IVC blood filter manufacturers, like Cook Medical and C.R. Bard, citing wrongful death and bodily injuries. IVC filter injury claims against other divisions include Cook Medical, LLC, Cook Group Incorporated, and Cook Medical. These lawsuits argue that implanted Cook IVC filters fractured inside their bodies, causing pieces to move through their bloodstream, causing adverse injuries.
Claims against Cook Medical and C.R. Bard were merged into a multidistrict litigation (MDL).
As of July 11, 2025, 6,978 lawsuits are still pending in the MDL 2570 before Senior Judge Richard Young in the Southern District of Indiana. A total of 11,448 lawsuits were filed.
C.R. Bard agreed to settlements in lawsuits against it in an Arizona federal court. The MDL 2641 against C.R. Bard was closed in July 2024, as all the C.R. Bard IVC blood filter lawsuits had been settled.
In various state courts and bellwether trials, juries delivered verdicts against Cook Medical and C.R. Bard. Bellwether trials, also known as test trials, are trials conducted in MDLs to establish if claimants have a case that may result in dismissals or settlements of the MDLs. Individual cases against other IVC blood clot filter manufacturers, including Argon, ALN, Cordis, Boston Scientific, and Rex Medical, are also still active.
IVC blood filter verdicts include:
| Date | IVC Filter Manufacturer | Plaintiff Verdicts |
| May 2018 | Cook Medical | In a Texas state court, a jury awarded Jeff Pavlock $1.2 million. He alleged that a Cook IVC filter damaged his small intestine and aorta. |
| February 2019 | Cook Medical | A jury had awarded $3 million to Tonya Brand for damages suffered when her Cook IVC filter ‘degraded’ inside her body. However, a judge overturned that verdict, and the lawsuit will go to trial again. |
| August 2020 | C.R. Bard | The 9th Circuit confirmed a $3.6 million jury award from 2028 to Sherr-Una Booker in an MDL bellwether trial. In five bellwether trials, this was the one time a claimant had won. |
| May 2021 | C.R. Bard | In Oregon, a jury awarded Justin Peterson $926,000. He had alleged that an IVC blood clot filter damaged his small intestines. |
Who Qualifies to File a Cook IVC Filter Lawsuit?
You may qualify to file a Cook blood clot filter lawsuit if you’ve sustained complications or injuries from an implanted Cook IVC filter. Claimants who have filed lawsuits against Cook Medical and other IVC filter manufacturers have listed thrombosis and embolization, filter fracture, migration, and perforation of veins among other health complications.
Talk to an attorney to determine if you meet the eligibility criteria to file an IVC filter lawsuit. Your attorney can assist you in collecting the necessary documents and navigating the legal process.
Steps to Take If You’ve Been Affected by an IVC Filter
If you have an IVC blood clot filter implanted, consult a doctor immediately to determine if the device should be retrieved. These medical devices are supposed to be temporary. So, having one in your body for too long can cause severe health complications.
And if you’re unsure about your device’s status or experience swelling, nausea, chest pain, fever, or redness after implantation, notify your doctor immediately.
Additionally, if an IVC filter has injured you, consider also talking to an attorney who specializes in defective medical device lawsuits to discuss your legal options.
Before speaking with a personal injury lawyer, gather your medical documentation, such as medical records about the IVC filter implantation procedure and any health complications you were treated for after the procedure. Acting quickly can minimize your health risks and help you understand your rights if a medical device fails.
At AllConsumer.com, we provide comprehensive consumer guides and reviews for a wide range of products and services. Sign up for our latest newsletter to stay informed.